Working group:
Myeloid Neoplasias
Supervisor: PD Dr. DI Lisa Pleyer
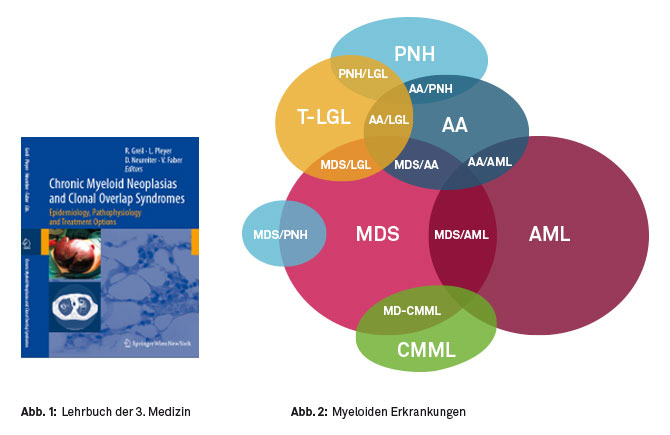
Since 2009 we have been investigating myeloid neoplasias, with the main focus on myelodysplastic syndromes (MDS), chronic myelomonocytic leukemia (CMML), and acute myeloid leukemia (AML), as well as various overlap syndromes (Figure 1). In 2010, we published an educational textbook on chronic myeloid neoplasias and overlap syndromes (Figure 2).
We founded a national patient registry of patients with MDS, AML and CMML treated with hypomethylating agents (HMAs). This registry is by now one of the largest and most well documented registries in this indication world-wide, and many fruitful national and international colaborations have resulted. This registry encompasses anonymous documentation of toxicities, response, and survival of patients with above mentioned diseases that are treated with HMAs.
In MDS, AML and CMML, so-called epigenetic changes are present and involved in disease pathogenesis. An example of how powerful epigenetics can be, is for example the transformation of a caterpillar to a butterfly. Both animals share the same genome (DNA sequence), but harbor different epigenetic changes, that result in the switching on or off of different genes, that can be ‘read’ and ‘translated’ into proteins. Epigenetic changes include DNA hypo- and hypermethylation, as well as methylation and acetylation changes of histone molecules, which may be described as spools, around which the DNA is wrapped. Depending on how the DNA is methylated, and how the histones are acetylated, the DNA can either be wrapped more tightly around the histones, resulting in a closed chromatin structure. In this closed state, tumor suppressor genes cannot be ‘read’ or ‘transcribed’, which may result in tumor initiation, propagation and/or progression. Treatment with HMAs results in a (partial) reversal of this hypermethylated state, and consequently leads to the unwrapping of DNA from the histones, and in a looser, more open chromatin structure. This is a state, where transcription factors can bind to the DNA and result in the reactivation and re-expression of tumor suppressor genes, among other effects. Thus HMAs can help eliminate myeloid cancer cells.
Our aim is to identify biomarkers, which may help predict which patients are the most likely to profit from treatment with HMAs. Other research interests of our group include the analyses of mutations and clonal evolution during the course of the diseases, as well as interactions of the malignant cells with various cells of the immune system, and, more specifically, the numerical changes of these cells during patient treatment with HMAs (Figure 3).
Effects of leukemic cells on immune cells
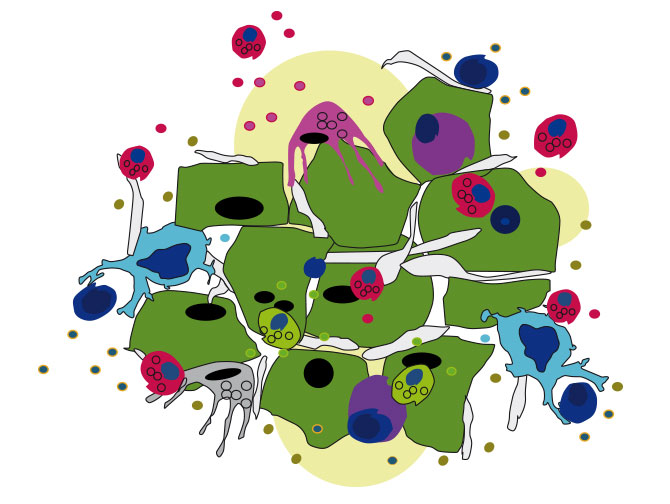
Leukemic cells modulate the microenvironment in general, and the immune system in particular, to create an environment, that allows the tumor to escape the control of the immune system (so-called ‘immune evasion’), and is permissive to further tumor cell growth. New targeted therapies, directed e.g. against certain checkpoint molecules on immune cells, can help reverse the tumor-induced micro-environmental changes. Ideally, these new drugs can change an immunosuppressive microenvironment to a tumor suppressive microenvironment, and thus ultimately help the bodies own immune system to eliminate cancer cells, or at least to control their growth.

Another part of our group is currently involved in establishing new techniques on how best to filter circulating cancer cells out of the peripheral blood, which will enable us to analyze tumor cells more closely in the future (Figure 4).

“Our aim is to better understand how we can use targeted immune therapies to help the body to eliminate myeloid cancer cells”
Team
PD Dr. DI Lisa Pleyer
(Supervisor)
OA Dr. Konstantin Schlick
Dr. Petra Altenhofer
(Biologist)
Dr. Thomas Barta
(Biologist)
Jakob Wagner, MSc
(Biologist)
Do you have a request or any questions?
Feel free to contact us – we will get back back to you as soon as possible.